My Projects
Topic: Gas Diffusion Electrodes GDE for Fuel Cells
Title: Production of GDE with Carbon Fibers
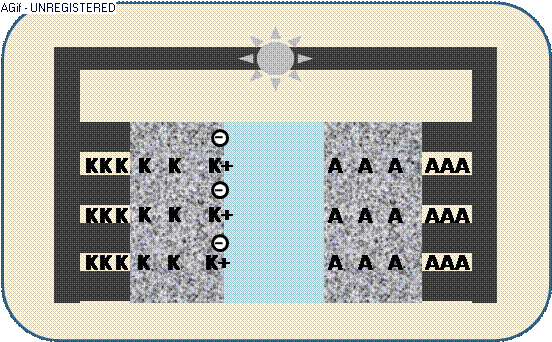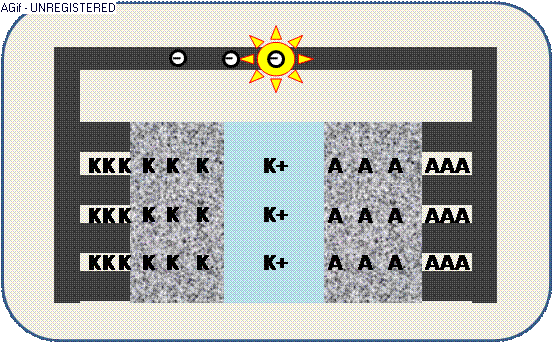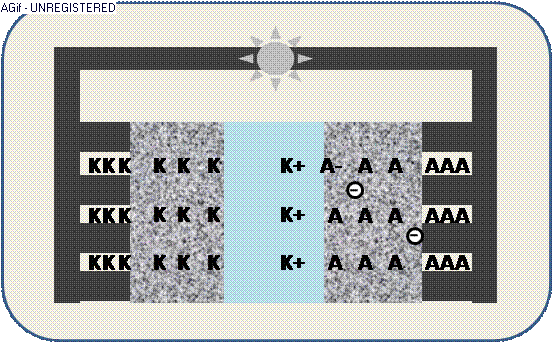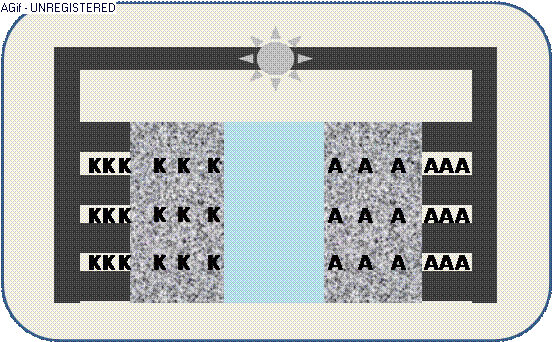
The animation illustrates the discharge within a galvanic cell:
By contact ions (+) are generated at the electrodes K/A and propagate through the electrolyte E.
The increased electric potential causes a circuit of electrons (-) from the collector,
where the generated electric current can be used by an appliance – e.g. a light emitter.
What one doesn’t see:
Galvanic cells are the basis for a direct conversion of chemical energy in an electric current. While a battery relies on the consumption of the electrode itself for the chemical reaction, a fuel cell requires a “fueling” agent to be transmitted through the electrode to the electrolyte. At the interface, the agent decomposes to cations (+) and anions (-) – generally enhanced by a catalyst – and reacting after passing the electrolyte, yet without burning.
Alternatively to the shown acid electrolyte there are also alkaline electrolytes, of course then carrying anions, respectively.
What’s behind it:
A fuel cell requires an equally highly porous and highly conductive layer for the electrode: the gas diffusion electrode or layer GDE or GDL, respectively. Furthermore, in regard to the caustic electrochemical media its material, the electrode material has to be highly resistant to corrosion.
Since the voltage of a galvanic cell is determined by the electrochemical potential of the fuel, the available power can just be increased by the amperage, i.e. the amount of reactions. And since the reactions rely on the available contact area, large-area constructions are required, such as stacks or wraps. In consequence, an industrial production of compact power packs needs particularly thin, smooth, flexible and bendable “papers” of porous, conductive and corrosion resistant material.
What you can obtain from it:
Originally, GDE is made on the basis of organic precursors – such as tar, pitch, fat, oil or wax – formed as thin films and then carbonized by a high-temperature curing process. The occurring evaporation provides the desired porosity and by carbonization the required electrical conductivity is achieved. However, the process is quite extensive – in cost and time – and the obtained carbon papers are rather fragile. This complicates the handling and a subsequent processing to a membrane electrode assembly MEA.
Alternatively, carbon paper can be produced on the basis of already carbonized fibers by a traditional paper making process. Especially, a dry “airlaid” process is appropriate to enable a paper structuring with desirable mean density, fiber orientation and stiffness. The airlaid process bears on the suspension of staple fibers in an upward airflow with a controlled settling velocity. For example, a surface structure of the paper with longer fibers of some millimeters can be filled up with an inner structure by short fibers of about ten to hundred microns.
The nonwoven is then fixed by a bonding agent or special bonding fibers. For example, a conductive nanocomposite employs a hydrophilic polysilane, which adjust itself at the junction of two crossing carbon fibers and thereby provides an equally chemical inert as well as electrical conductive composite.
Such papers are fabricable as endless blanks with considerable flexibility and a flexural strength as any other pulped paper. By simple tailoring the manufacturing of large area GDE for fuel cells becomes available – simplified, automatable and ready for mass production.
My related publications:
Multilayered, Flexible Paper Containing Carbon, with Good Flexual Strength, patent US 20040053112 A1 (2004)
Fuel Cell, patent WO 2003096451 A2 (2003)